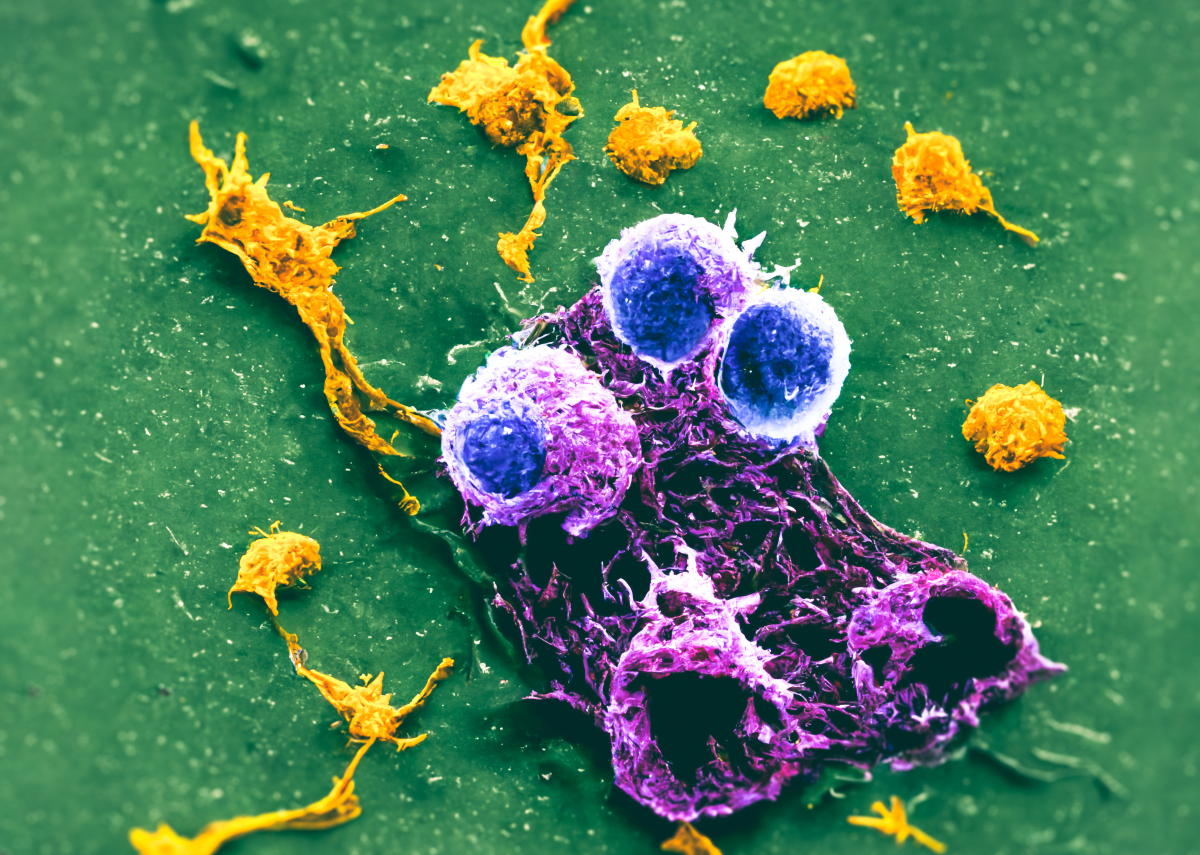
Penetration of nano-agent into cancer cells

How would you feel if you were told that there are about 8 billion libraries, each with about 46 bookshelves and a total of 20,000 to 25,000 books? Each library is estimated to contain about 3 billion pages of paper.
MSTF media reports:
This incredible example represents us humans. The library indicates the genome that holds all the essential information for human growth. Currently, as we read this, the world's population is about 8 billion people, each possessing 46 chromosomes. A chromosome, like a shelf of books, contains about 20,000 to 25,000 “books”, which in biological terms are referred to as genes. A gene is the functional segment of DNA that holds the instructions for producing proteins. But the story does not end there. Each gene is made up of parts called introns and exons. The exon is the part that remains after splicing and serves as a basis for protein expression. Introns, on the other hand, undergo splicing during transcription and are subsequently deleted. As a result, they don’t exist in the RNA. Notably, the human genome is said to contain 3 billion nucleotide pairs!
By performing other calculations with the same numbers, one can reach even more surprising figures and gain insights into the wonders of our world. In this report, we will examine Omid Farokhzad's achievements and talk about his research priorities.
Over the past 20 years, technological advances have enabled deep and broad access to genomic information. The collective efforts of the genomics community have resulted in the sequencing of over 1 million human genomes and over 10 million human exomes (the collection of all the exons within the genome). Across these efforts, scientists have identified over 1.3 billion genetic variants. Genetic variants are different forms of a gene that cause differences among individuals. Nevertheless, we know very little in terms of the functional importance of these genomic variants.
Ultimately, the information contained in DNA is converted into proteins, creating a new term called proteomes. The proteome is a set of all expressed proteins in a cell, tissue, or organism. Understanding the importance and complexity of the proteome paves the way for future advances in biology while the role of curious scientists seeking a deep understanding of the proteome becomes more prominent.
But why is it important to analyze these small yet impactful details? By studying the proteome in a deep and detailed manner it is expected that we’ll identify the reason for the difference among the one million proteins that are produced by approximately 20,000 genes. Such variety has different reasons. Differences can be the result of amino acid changes or changes caused by RNA splicing, or even changes following translation. Studies conducted to better understand proteins are essential and ultimately provide a range of applications for biologists. One of the key applications is our enhanced understanding of biology which will ultimately help us find new biomarkers for diseases. A biomarker is a biological molecule found in blood, other body fluids, or tissues and is a sign of normal bodily function, or of an abnormal condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease. Biomarkers have significantly contributed to the timely diagnosis (detecting cancer in the early stages), monitoring (observing the response to cancer treatments and assessing treatment effectiveness) and prediction (prevention and early diagnosis) of diseases. They assist doctors and researchers in the diagnosis and monitoring of prognostic conditions and stages of diseases.
Proteome Helps New Drugs Kick In
Examining other applications of biomarkers in the area of disease monitoring will lead to the discovery of new drugs. Studying proteins enables scientists to create new potential drug targets. Molecules or new proteins can be used as a target for developing new drugs. These discoveries open up new markets in the field of biology, which, in addition to having a positive impact on public health, are also economically beneficial. An important achievement that is a result of the convergence of all three applications of proteomic studies is the role of nanoparticles in therapy.
The Leverage to Beat Cancer
Among the most important applications of nanoparticles is the treatment of the emperor of all diseases, cancer. Cancer is one of the greatest medical challenges, and common treatments such as chemotherapy and radiation therapy have numerous side effects. Our trump card against cancer is the magical particles called nanoparticles. The importance of these particles can be examined from several aspects. Crossing biological barriers, precise targeting of cancer cells, drug delivery to specific body parts and maintaining the health of non-cancerous tissues are among the properties of nanoparticles that bring great hope to scientists and patients.
You may wonder what the connection between the proteome, cancer treatment, and nanoparticles is. The answer to this question lies in Omid Farokhzad's research findings. This question was also answered at the 23rd Science Café the main focus of which was the use of nanoparticles in cancer treatment. The guest of this session, a bioinformatics professor at Tehran University, introduced the distinctive features of nanoparticles and the challenges of using them.
“The body's biological system is very complex, and every molecule or particle injected into the body must be able to pass through the lipophilic coating of cells if necessary. On the other hand, despite their high effectiveness, a number of drugs cannot dissolve well in aqueous environments or flow through the blood due to their high lipophilicity. Therefore, the use of ‘smart nanoparticles or carriers’ in therapeutic methods becomes important,” reviewing Farokhzad's achievements.
“Since nanocarriers are extremely accurate in identifying the target cells and releasing drugs directly in that area, using them for targeted drug delivery to the location of cancer cells can greatly reduce the side effects that come with common methods of treating this disease,” he added, pointing out the need to design new treatment methods with fewer side effects. In his research, Farokhzad has shown that specific conditions must be provided when releasing drugs at the target site.
According to these studies, in order for nanoparticles to be able to identify cancer cells, specific functional groups must be placed on them. For example, folic acid—recognized by specific receptors on ovarian cancer cells—can bind specifically to the target cell and release the drug. In some cancers, specific proteins are expressed in target cells; therefore, nanoparticles must be designed to bind to these proteins and release the drug.
Such discoveries result from paying attention to small, yet impactful details in biology such as studying the proteome. Technological advances over the past 5 years have enabled access to deep unbiased proteomics at an unprecedented scale. These advances include the convergence of the Proteograph Product Suite that enables deep unbiased sampling of the proteome with next-generation mass spectrometers that are faster and more sensitive. It also enables precise detection of proteins and their variants at the peptide level resolution. Our current knowledge has only scratched the surface of the proteome. We may have gained few insights into nanoparticles and cancer but the iceberg remains to be discovered.
"This material was originally published in the second issue of the International Observatory Journal."